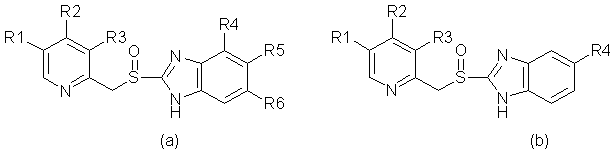
Figure 1. (a) Basic structure for omeprazole and its derivatives;
(b) Basic structure for 2-[[(2-Pyridyl)methyl]thio]-1H-benzilidazoles;
Rn = substituent.
Bruni A. T., da Silva A. B. F., Ferreira M. M. C., Leite V. B. P., "Conformational Analysis of Omeprazole Family Compounds". Caxambu, MG, 23-26/11/2003: 12° Simpósio Brasileiro de Química Teórica (XII SBQT) [12th Brazilian Symposium of Theoretical Chemistry], Livro de Resumos [Book of Abstracts], (2003) P013. Poster P013
|
|
CONFORMATIONAL
ANALYSIS OF OMEPRAZOLE FAMILY
COMPOUNDS
Aline
T. Bruni1
(PQ), Albérico B. F. da Silva2 (PQ),
Márcia M. C. Ferreira3 (PQ),
Vitor
B. P. Leite1
(PQ)
alinetb@df.ibilce.unesp.br
1DepartamentodeFísica-IBILCE-Institutode
Biociências,LetraseCiênciasExatas,Universidade
Estadual Paulista, São
José do Rio Preto - SP 15054-000, Brasil -
2DepartamentodeQuímicaeFísicaMolecular,InstitutodeQuímicadeSão
Carlos,Universidadede
São Paulo, Av.
do Trabalhador São-Carlense, 400 - Centro, São Carlos - SP
13560-970, Brasil
3InstitutodeQuímica-UniversidadeEstadualdeCampinas,CidadeUniversitária"Zeferino
Vaz",
Barão Geraldo
- Caixa Postal 6154 - Campinas - SP 13084-971, Brasil
Keywords: omeprazole, chemometrics, conformational analysis
Many problems in theoretical
medicinal chemistry studies requires
previous
conformational analysis
before evaluating the properties of a given set of molecules regarding
to
their biological
activity. In this work,
two sets of compounds with anti-Heliobacter
pylori
(bacterium responsible for
peptic ulcers) activity were studied. The
former set is omeprazole
and its derivatives,
and the last one is the sulfide precursor of omeprazole and
analogues (2-
[[(2-Pyridyl)methyl]thio]-1H-benzilidazoles),
their basic structures are shown in Figure 1:

Figure
1. (a) Basic structure for omeprazole and its derivatives;
(b)
Basic structure for 2-[[(2-Pyridyl)methyl]thio]-1H-benzilidazoles;
Rn
= substituent.
Conformational analysis was performed using a method
which finds minimum energy
structures.
This method associates principal component
analysis with quantum mechanic
calculations.
It controls the combinatorial explosion inherent
to the conventional systematic
search by a
dimensional reduction of the system
through the use of principal component
analysis [Bruni AT,
Leit VBP, Ferreira MMC, J. Comp. Chem. 2002; 23: 222]. As
result, it was
observed that
small differences among the compounds inside
each group provide important
changes in the minimum
energy structures. Comparisons between the two
sets of compounds
showed that the presence
of a S=O group for omeprazole and its derivatives
was responsible
for significant differences
from the respective precursor sulfide and analogues minimum
energy
structures.
The most important conclusion is observed when the number
of minimum energy
structures for basic structure
and for the other molecules are compared. If the
calculation was
performed only on the basic
structure, followed by the substituents addition,
all the molecules
would have only
two minimum energy structures (number
found for both basic structures,
Fig. 1). Nevertheless,
the addition of each substituent prior
the complete conformational
analysis calculations is
crucial in these studies, because, despite of all minimum
conformations
have similar energetic values,
some calculated properties used for further SAR/QSAR
studies
can be very sensitive
to small structural variations [Bruni AT, Ferreira MMC, J.
Chemom. 2002;
16: 510]. (FAPESP/CAPES/CNPq).