Ferreira M. M. C., Kiralj R., Vencato I., "A Study of 4-(E)-Amino-3[(E)-nitrophenylazo]-3-penten-2-one and Other Related Azoenaminones in Crystalline State". Caxambu, MG, 23-26/11/2003: 12° Simpósio Brasileiro de Química Teórica (XII SBQT) [12th Brazilian Symposium of Theoretical Chemistry], Livro de Resumos [Book of Abstracts], (2003) P148. Poster P148.
|
|
A
STUDY OF 4-(E)-AMINO-3-[(E)-4-NITROPHENYLAZO]-3-
PENTEN-2-ONE
AND OTHER RELATED AZOENAMINONES IN
CRYSTALLINE
STATE
Márcia
M. C. Ferreira (PQ),1
Rudolf Kiralj (PQ),1 Ivo Vencato (PQ).2
marcia@iqm.unicamp.br
1Instituto
de Química - UNICAMP, Campinas SP, 13084-971 Brazil. 2Instituto
de Física - UFG,
Goiânia
GO, 74001-970, Brazil.
Palavras-Chaves: ab initio, molecular and crystal structure, chemometrics
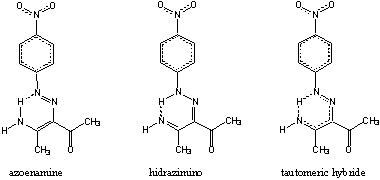
Azoenaminones are compounds with potential nonlinear
optical properties [1]. They
exist as azoenamine/hidrazimino
tautomeric mixtures in solution (Scheme). There
are only a
few structural analyses
of 4-nitrophenylazoenaminones and related compounds [2-4], which
do
not provide a clear
picture of tautomeric composition of these compounds
in crystalline state.
This is the
reason to study
more profoundly the
nature of 4-
nitrophenylazoenaminones,
and particularly of 4-(E)-amino-3-[(E)-4-nitrophenylazo]-3-penten-2-
one by ab initio
(B3LYP 6-31G**) methods in free and complexed state, by making
structural
comparison of related crystal
structures retrieved from the Cambridge Structural Database, and
by chemometric
analysis on molecular and crystal structure
parameters for the compared
phenylazoenaminones.
The title and other similar compounds in free state
represent planar heteroaromatic
systems where
tautomerism is coupled with intrinsic p-electron
delocalization (inside the
nitrobenzene
and azoenamine fragments, and
between them and the keto group) and
intramolecular hydrogen
bonds (Scheme). Based on calculated and experimental bond lengths
and bond orders, the
compounds do not show the same tautomeric
character in crystalline
state. N-N
bond length seems to be a reliable
parameter for monitoring the p-electron
delocalization and
aromaticity change due to electron withdrawal/donation by
substituents on
phenyl and/or
enamine fragment. Intermolecular hydrogen bonds,
dipole-dipole and other
intermolecular interactions
in crystal affect molecular geometry, particularly planarity,
and are
partially responsible for
crystal packing. Some electronic and steric molecular properties
exhibit
qualitative or
quantitative relationship with crystal packing
properties like crystal density,
coordination number,
average atomic volume, and ability to form hydrogen bond networks
or
p…p
stacks. Molecular structure of an azoenaminone in crystal
is mainly located close to a
delocalized tautomer,
what does not always provide reliable experimental determination
of its
tautomeric composition
in solid state. (FAPESP and CNPq)

Scheme. Tautomeric/resonance forms of the title compound in crystalline state
[1] L. J. O. Figueiredo,
C. Kascheres, J. Org. Chem. 62 (1997) 1164.
[2] B. L. Rodrigues et al.,
Acta
Cryst. C52 (1996) 705.
[3] V. Kettmann et al.,
Acta
Cryst. C57 (2001) 737.
[4] P. Šimunek et al., J.
Mol. Struct. 642 (2002) 41.