Ferreira M. M. C., Kiralj R., "QSAR and Conformational Study of 1H-Indole-3-Acetic Acids with Auxin Activity". Caxambu, MG, 23-26/11/2003: 12° Simpósio Brasileiro de Química Teórica (XII SBQT) [12th Brazilian Symposium of Theoretical Chemistry], Livro de Resumos [Book of Abstracts], (2003) P150. Poster P150.
|
|
QSAR
AND CONFORMATIONAL STUDY OF 1H-INDOLE-3-
ACETIC
ACID WITH AUXIN ACTIVITY
Márcia
M. C. Ferreira (PQ), Rudolf Kiralj (PQ)
rudolf@iqm.unicamp.br
Instituto
de Química - UNICAMP, Campinas SP, 13084-971 Brazil.
Palavras-chave: QSAR, auxins, chemometrics
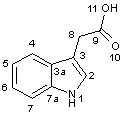
1H-Indole-3-acetic acid (IAA, Scheme)
and its derivatives are among the
most
important plant-growth
regulation hormones from auxin class. Their
Quantitative Structure-
Activity Relationships
(QSAR) are not straitforward, mainly due to the
lack of 3D structure of
a receptor which has been
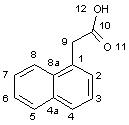
determined just recently for a complex of auxin ABP1 (auxin binding
protein 1) with 1-naphthalenic
acid (NAA, Scheme), and intrinsic auxin properties. IAA consists
of planar heteroaromatic
indole ring, and the side chain acetate group which can adopt
a few
distinct orientations
with respect to the ring. Usually IAA auxins are limited
to derivatives with
small substituents
at five substitution positions at the indole ring.
This work deals with QSAR and conformational properties of 22 IAAs,
11/15 of which
has measured
straight-growth promoting activities on Avena
Sativa L. coleoptiles [1]: the
optimal coleoptile elongation
L,
the half-optimum concentration c, and the optimal concentration
m, all
relative to IAA. Conformers close to IAA in crystalline
state were selected for geometry
optimization
at ab initio B3LYP 6-31G** and
MMFF94 level, and various topological,
electrotopological,
steric, electronic and lipophilicity molecular
descriptors were calculated.
Selected descriptors were
then analyzed by means of Hierarchical Cluster Analysis (HCA)
and
Principal Component Analysis
(PCA). Partial Least Squares (PLS), Principal Component (PCR)
and Multiple Linear
(MLR) regression models were built and validated for estimation/prediction
of the biological activities
logL, logc and logm. Twenty-two ABP1 auxin complexes
built from
ABP1 NAA complex
crystal structure [2], and their geometries
optimized by MMFF94.
The regression models were based on
11 (alkylated) and 15 (alkylated
and
fluorinated) IAAs in the
training set. In general, PLS and PCR models are
similar, while MLR
models are worse.
Predicted activities agree reasonable
with expectations and current
knowledge on IAA auxin activity.
Molecular graphics on ABP1-NAA/IAA complexes give
more
mechanistic insight into
ABP1 auxin binding, which is specially related to
logc. HCA and
PCA analyses
show discrimination of the auxins with respect
to their activity and molecular
characteristics. Conformation
of an IAA molecule regarding to the CH2CO2H
side chain plays a
very important rule in
IAA behavior. Remarkable differences in torsion angles
T1 (C2-C3-C8-
C9) and
T2 (C3-C8-C9-O10) can be observed for IAA in free state,
in its crystal and some
crystals of molecular complexes
including IAA, and in modeled ABP1 IAA complex.
Besides,
due to complexation of Zn2+
ion from ABP1 with IAAs, T1 and T2 show systematic changes with
respect to free or crystalline
state of these IAAs. T1 and T2 are
related to biological activities
and molecular properties
at qualitative and even quantitative level. (FAPESP)


Scheme. 1H-indole-3-acetic acid and 1-naphthalenic acid
[1] B. Nigovic et al., Acta
Cryst. B56 (2000) 94.
[2] E.-J. Woo et al., EMBO
J. 21 (2002) 2877.